Medical Device Ifu Example . Web which medical devices require instructions for use? The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. Ce mark for medical device. Web medical devices can be complex and may require users to understand specific information about storage, use, disposal, or reprocessing. Form of prescription drug labeling. Instruction for use (ifu) under the eu mdr (eu 2017/745) and ivdr (eu 2017/746) , all medical devices must have an instructions. Web what is an instruction for use (ifu) document? Instructions for use (ifu) is: Poor instructions for use are a common cause of use errors that can result in harm to patients and users. In the eu, and according to the medical device regulation (2017/745 mdr annex i, 21.1 d)), class i and.
from www.freseniusmedicalcare.com
Web which medical devices require instructions for use? Form of prescription drug labeling. Instruction for use (ifu) under the eu mdr (eu 2017/745) and ivdr (eu 2017/746) , all medical devices must have an instructions. Poor instructions for use are a common cause of use errors that can result in harm to patients and users. In the eu, and according to the medical device regulation (2017/745 mdr annex i, 21.1 d)), class i and. Ce mark for medical device. Web what is an instruction for use (ifu) document? The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. Web medical devices can be complex and may require users to understand specific information about storage, use, disposal, or reprocessing. Instructions for use (ifu) is:
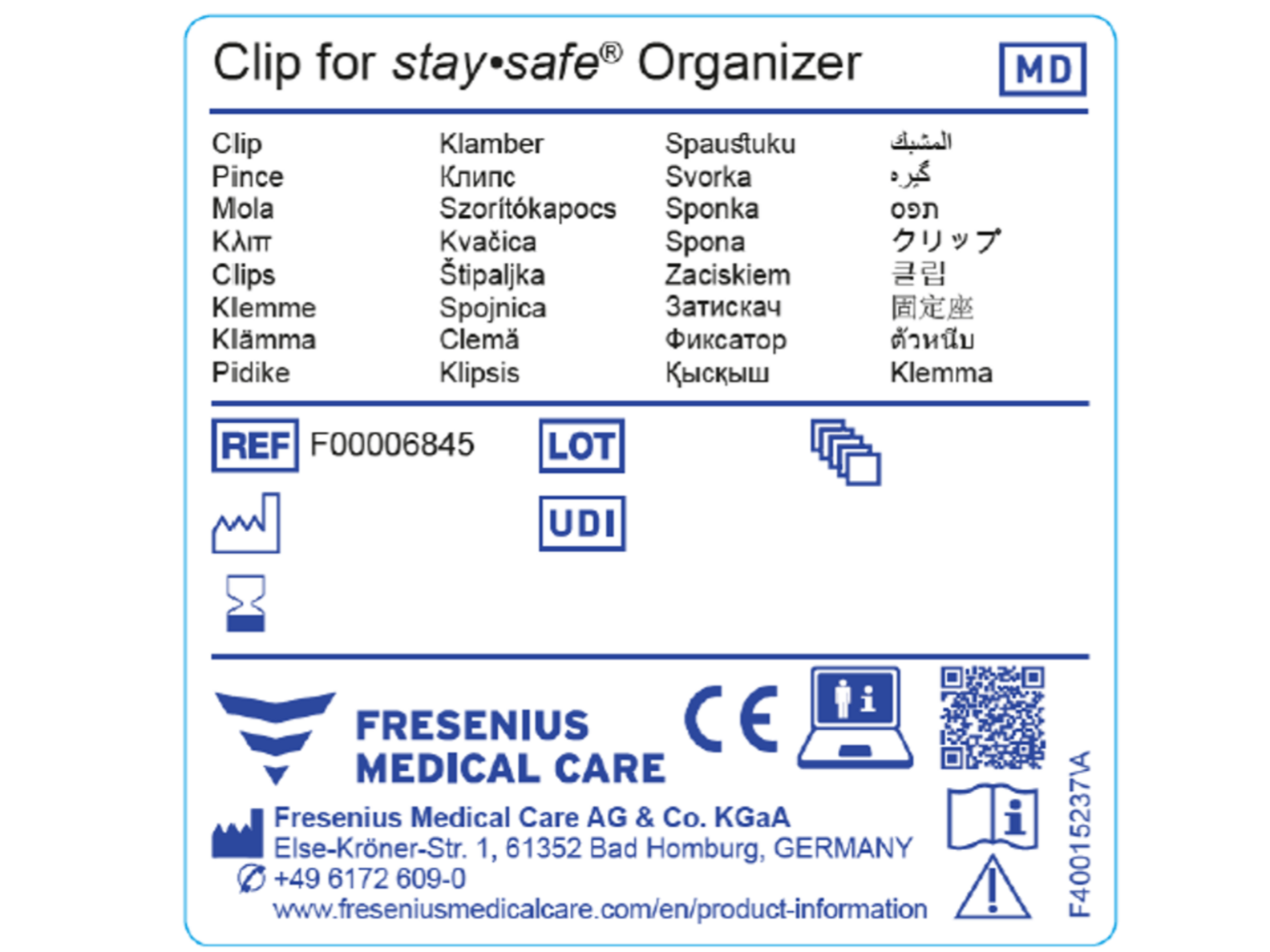
MedizinprodukteVerordnung Fresenius Medical Care
Medical Device Ifu Example Form of prescription drug labeling. In the eu, and according to the medical device regulation (2017/745 mdr annex i, 21.1 d)), class i and. Form of prescription drug labeling. Web which medical devices require instructions for use? Instructions for use (ifu) is: Poor instructions for use are a common cause of use errors that can result in harm to patients and users. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. Web medical devices can be complex and may require users to understand specific information about storage, use, disposal, or reprocessing. Instruction for use (ifu) under the eu mdr (eu 2017/745) and ivdr (eu 2017/746) , all medical devices must have an instructions. Web what is an instruction for use (ifu) document? Ce mark for medical device.
From easymedicaldevice.com
How to Create a Label as per EU MDR 2017/745? Medical Device Ifu Example Form of prescription drug labeling. Web what is an instruction for use (ifu) document? The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. Instruction for use (ifu) under the eu mdr (eu 2017/745) and ivdr (eu 2017/746) , all medical devices must have an instructions. Instructions for use (ifu) is: In the eu,. Medical Device Ifu Example.
From www.vrogue.co
Fda Medical Device Labeling Requirements Presentation vrogue.co Medical Device Ifu Example Web which medical devices require instructions for use? The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. Web what is an instruction for use (ifu) document? Ce mark for medical device. In the eu, and according to the medical device regulation (2017/745 mdr annex i, 21.1 d)), class i and. Instruction for use. Medical Device Ifu Example.
From www.appliedmedical.net
Device Instructions for Use Applied Medical Technology Medical Device Ifu Example Form of prescription drug labeling. Web medical devices can be complex and may require users to understand specific information about storage, use, disposal, or reprocessing. Ce mark for medical device. Web which medical devices require instructions for use? Instructions for use (ifu) is: Instruction for use (ifu) under the eu mdr (eu 2017/745) and ivdr (eu 2017/746) , all medical. Medical Device Ifu Example.
From medicaldeviceacademy.com
IFU validation is not a risk reduction Deviation 7 Medical Device Ifu Example Instructions for use (ifu) is: Web what is an instruction for use (ifu) document? Ce mark for medical device. Form of prescription drug labeling. Instruction for use (ifu) under the eu mdr (eu 2017/745) and ivdr (eu 2017/746) , all medical devices must have an instructions. Poor instructions for use are a common cause of use errors that can result. Medical Device Ifu Example.
From pdihc.com
Prevantics® Device Swab Instructions For Use (IFU) Sign PDI Healthcare Medical Device Ifu Example Form of prescription drug labeling. Web what is an instruction for use (ifu) document? Instructions for use (ifu) is: In the eu, and according to the medical device regulation (2017/745 mdr annex i, 21.1 d)), class i and. Poor instructions for use are a common cause of use errors that can result in harm to patients and users. Web medical. Medical Device Ifu Example.
From instrktiv.com
IFU for Medical Devices, a Definitive Guide (EU & US) Medical Device Ifu Example Web medical devices can be complex and may require users to understand specific information about storage, use, disposal, or reprocessing. Poor instructions for use are a common cause of use errors that can result in harm to patients and users. Form of prescription drug labeling. Ce mark for medical device. The purpose of this imdrf guidance is to provide globally. Medical Device Ifu Example.
From eo2.com
Instructions For Use (IFU) Innovative Wound Healing EO2 Concepts Medical Device Ifu Example The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. Web medical devices can be complex and may require users to understand specific information about storage, use, disposal, or reprocessing. Ce mark for medical device. Poor instructions for use are a common cause of use errors that can result in harm to patients and. Medical Device Ifu Example.
From instrktiv.com
IFU Medical Device Create Instructions For Use Medical Device Ifu Example Instruction for use (ifu) under the eu mdr (eu 2017/745) and ivdr (eu 2017/746) , all medical devices must have an instructions. Ce mark for medical device. In the eu, and according to the medical device regulation (2017/745 mdr annex i, 21.1 d)), class i and. Web which medical devices require instructions for use? The purpose of this imdrf guidance. Medical Device Ifu Example.
From pinterest.com
Image of medical device quick reference guide IFUs Pinterest Medical Device Ifu Example The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. Ce mark for medical device. Web which medical devices require instructions for use? Web medical devices can be complex and may require users to understand specific information about storage, use, disposal, or reprocessing. Poor instructions for use are a common cause of use errors. Medical Device Ifu Example.
From eifu-service.com
US FDA Guidance on IFUs for Medical Devices EIFU Portal Medical Device Ifu Example Web which medical devices require instructions for use? Form of prescription drug labeling. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. Web what is an instruction for use (ifu) document? In the eu, and according to the medical device regulation (2017/745 mdr annex i, 21.1 d)), class i and. Ce mark for. Medical Device Ifu Example.
From www.diamondortho.com
IFU's Medical Device Ifu Example The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. Web what is an instruction for use (ifu) document? Poor instructions for use are a common cause of use errors that can result in harm to patients and users. Web which medical devices require instructions for use? Ce mark for medical device. Instruction for. Medical Device Ifu Example.
From www.royallabel.com
IFUs for Medical Devices and Pharmaceuticals IFU Printing Services Medical Device Ifu Example Poor instructions for use are a common cause of use errors that can result in harm to patients and users. The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. Instruction for use (ifu) under the eu mdr (eu 2017/745) and ivdr (eu 2017/746) , all medical devices must have an instructions. Web what. Medical Device Ifu Example.
From instrktiv.com
IFU for Medical Devices, a Definitive Guide (EU & US) Medical Device Ifu Example The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. Instruction for use (ifu) under the eu mdr (eu 2017/745) and ivdr (eu 2017/746) , all medical devices must have an instructions. Web what is an instruction for use (ifu) document? Instructions for use (ifu) is: Ce mark for medical device. Poor instructions for. Medical Device Ifu Example.
From custom-medical.com
IFU Design & Testing Custom Medical Medical Device Ifu Example Poor instructions for use are a common cause of use errors that can result in harm to patients and users. Web which medical devices require instructions for use? Ce mark for medical device. Web what is an instruction for use (ifu) document? Instructions for use (ifu) is: The purpose of this imdrf guidance is to provide globally harmonized labelling principles. Medical Device Ifu Example.
From testlabsuk.com
IFU validation requirements for Class Ir Medical Devices Test Labs Medical Device Ifu Example The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. Instruction for use (ifu) under the eu mdr (eu 2017/745) and ivdr (eu 2017/746) , all medical devices must have an instructions. Poor instructions for use are a common cause of use errors that can result in harm to patients and users. Ce mark. Medical Device Ifu Example.
From www.linkedin.com
Medical Device IFU & eIFU Medical Device Ifu Example Ce mark for medical device. In the eu, and according to the medical device regulation (2017/745 mdr annex i, 21.1 d)), class i and. Poor instructions for use are a common cause of use errors that can result in harm to patients and users. Form of prescription drug labeling. The purpose of this imdrf guidance is to provide globally harmonized. Medical Device Ifu Example.
From www.behance.net
IFU Medical Robotics Device on Behance Medical Device Ifu Example Ce mark for medical device. Instructions for use (ifu) is: Web medical devices can be complex and may require users to understand specific information about storage, use, disposal, or reprocessing. Web what is an instruction for use (ifu) document? The purpose of this imdrf guidance is to provide globally harmonized labelling principles for medical devices,. Form of prescription drug labeling.. Medical Device Ifu Example.
From exogphupj.blob.core.windows.net
Medical Device Labelling Tga at William Maurer blog Medical Device Ifu Example Web which medical devices require instructions for use? Poor instructions for use are a common cause of use errors that can result in harm to patients and users. Web what is an instruction for use (ifu) document? Instructions for use (ifu) is: In the eu, and according to the medical device regulation (2017/745 mdr annex i, 21.1 d)), class i. Medical Device Ifu Example.